An Oregan woman has warned professionals of the risks of taking CBD oil after losing an important job offer as a nurse.
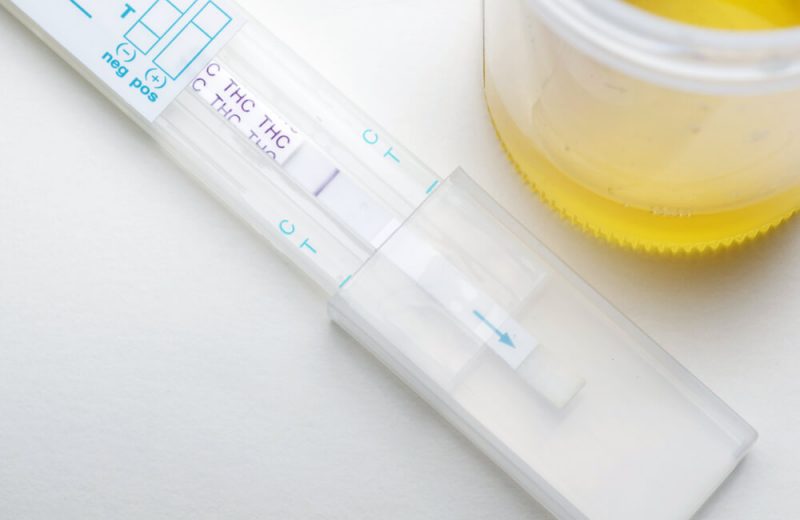
Suzan Chandler, a nurse practitioner from Hillsboro, Oregon was up for a job at her local urgent care facility when she lost the opportunity following a drug test. The nurse tested positive for tetrahydrocannabinol (THC), a product found in high concentrations in marijuana, responsible for the drug’s psychoactive effects.
Chandler said she had been taking hemp protein and CBD for its calming effects, but that she had never touched marijuana.
CBD products, although derived from the cannabis plant, are only legally allowed to contain 0.3% of THC.
Speaking to Western Mass News, Chandler said, “Tears. I never thought, I never used a product knowingly with THC, I wouldn’t.”
Renee Barnes, co-owner of the Portland Store CBD-Lish told the news outlet of the importance of doing a little extra research on any CBD product before buying, whether it’s oil, a cream, or even just a sheet mask.
She said, “We’ve had firemen, we’ve had policemen, we’ve had people doing jobs that are very, you know, essential that they don’t [take THC-containing products] and you know we’ll talk to them about it.”
Chandler added a warning to others, saying, “We get drug screened for a reason and those are good reasons. They just have to treat all the employees that test positive the same.”
“Our family all of a sudden doesn’t have my income. It’s a significant income change. So, after all that wave, I decided that I wanted to make sure that other people could learn that and not have to go through what our family has.”
It has become more and more of a concern in recent months that consumers aren’t sure what CBD product they’re purchasing when walking into a store.
In the last week, the FDA has warned 15 companies they are illegally selling CBD products – either marketing them as health supplements, adding them to food, or claiming unproven therapeutic effects.
So far, the health agency has only approved Epidiolex, a CBD-containing drug, to treat two forms of rare and severe epilepsy.
The FDA doesn’t fully regulate CBD products at present, leading to concern from experts about exactly what is on the market.
CBD tinctures, for example, come in both full- and broad-spectrum forms. A label advertising a product as full-spectrum contains THC, which shouldn’t be more than 0.3%, but worries have arisen that some companies may be misleading consumers.
There are also significant differences between hemp oil and CBD oil, which some remain unaware of.
Barnes pointed out that taking a full-spectrum product doesn’t necessarily mean the user will fail a drug test, but better to be safe than sorry.
While CBD use is accepted in many occupations, it remains important that customers shop at places where employees are knowledgeable and can explain the product they are buying to them.